Orthosilicic acid exists in drinking water. This is an idealised presentation because of polymerisation reactions. In practice, analysts measure “reactive silica.”
Well – that’s what we can infer from a new campaign of the Fluoride Action Network (FAN).
But what is the link with fluoride – the whole reason for FAN’s existence? Well, they base this campaign on the well-known hydrolysis of the fluorosilicates used in community water fluoridation to form the hydrated fluoride anion and silica. (Although these campaigners are confused here as they will also often claim fluorosilicates do not hydrolyse and survive to come out of your tap and poison you).
Then they claim that “silicic acid” (silica in solution) dissolves lead from the pipes and fittings and this lead causes brain damage. A double-barreled danger as they also claim the fluoride also damages your brain. What’s more – they also claim that “silicic acid” may dissolve your teeth!
But there are two problems with this:
- Your drinking water contains silica whether it is fluoridated or not. So their warnings about the silica in fluoridated water should also be valid for “fluoride-free” water which they promote!
- They do not have a viable chemical mechanism for silica dissolving your pipes (and there are plenty of other mechanisms which can result in corrosion of pipes anyway). The same for your teeth. This claim is just not supported by the chemical literature.
I will just concentrate here on the “evils” of silica (or “orthosilicic acid”) that are being promoted by FAN and leave the lead story for another day. These “evils” all come down to concepts being promoted by Richard Sauerheber who FAN describes as “the ultimate citizen chemist.” (OK, he is their ultimate citizen chemist). His argument is presented in Silicic Acid – How Does Fluorosilicic Acid Leach Lead? Why Does Fluorosilicic Acid Leach Lead So Much More Than Sodium Fluoride?
Is silicic acid the bogy Sauerheber claims?
His article is confused and convoluted. But it starts with the assumption that silica in drinking water (silicic acid or orthosilicic acid) is bad. He declares:
“Neither fluoride nor silicic acid are constituents of normal pristine human or mammalian blood, but rather are contaminant materials, . . “
And he states:
“The mass treatment of public fresh drinking water with industrial fluorosilicic acid to produce fluoride ion at 1.0 ppm also produces approximately 6 ppm sodium ion and .7 ppm orthosilicic acid. None of these is found in or belongs in fresh drinking water.”
Let’s stop right there and check out his claim that sodium and “orthosilicic acid” are not found in fresh drinking water.
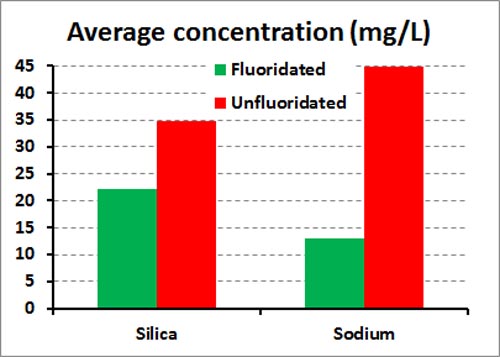
Here is some data for drinking water in fluoridated and unfluoridated areas of Auckland, New Zealand, taken from the WATERCARE ANNUAL WATER QUALITY REPORT 2013. The data are for 15 unfluoridated treatment plants and 8 fluoridated treatment plants.
These are average figures over all the plants. But values for silica as high as 64 mg/L in the unfluoridated plants and 44 mg/L in the fluoridated plant were recorded. The corresponding figures for sodium were 140 and 22 mg/L.
So Sauerheber is completely wrong. Unfluoridated water does contain silica and sodium. And at concentrations much higher than could be accounted for by added fluoridating chemicals – he calculated 0.7 mg/L – 0.7 ppm) for silica. In fact, the values are higher for the unfluoridated treatment plants in these examples.
Fluoridation makes a minuscule contribution to the concentration of these chemical species in drinking water.
Forget about fluoridation. If silica (and sodium) are such problems then Sauerheber should be campaigning against unfluoridated water as well. Even the “pristine” water in his local river or spring – silica is a normal and natural component of surface and bore waters.
But Sauerheber is also wrong about the dangers of silica in drinking water. Of course silica is present in “normal pristine human or mammalian blood” because it is part of our diet (Bisse et al 2005). It is a component of many of our foods. Sauerheber is simply attempting to confuse the issue because of the current lack of knowledge about the role of silica in the body.
But Jugdaohsingh et al., (2015) say:
“Silicon (Si) is a natural trace element of the mammalian diet and although it has not been demonstrated unequivocally that mammals have a requirement for Si there is increasing evidence to suggest that it may be important for the normal health of bone and the connective tissues. Indeed, severe dietary Si deprivation in growing animals appears to cause abnormal growth and defects of the connective tissues”
Given that silica appears to be important the presence of it in our drinking water is as an advantage. According to Jugdaohsingh (2007):
“Drinking water and other fluids provides the most readily bioavailable source of Si in the diet, since silicon is principally present as Si(OH)4, and fluid ingestion can account for ≥ 20% of the total dietary intake of Si.”
One trick Sauerheber uses is to cite reports of the danger of inhaled silica dust – which can cause cancers and silicosis. Completely irrelevant because of its different chemical form. This is equivalent to the trick often used by anti-fluoride campaigners of citing reports of results of industrial pollution or studies from areas of endemic fluorosis to support their attacks on community water fluoridation.
Will silica dissolve your teeth?
This claim is completely unsupported – no citations and purely a figment of Sauerheber’s imagination. He claims:
“Orthosilicic ‘weak’ acid has been long used in agriculture to break down solid calcium phosphate Ca3(PO4)2, thereby releasing soluble phosphate ion in soils even at neutral pH, for uptake by plant life. The reaction of silicic acid with calcium phosphate under neutral pH conditions is:
H4SiO4 + Ca3(PO4)2 → HPO4-2 + 3Ca2+ + PO4-3+ H3SiO4–.
This reaction occurs at a pH where any strong acid would have been neutralized. Orthosilicic acid is reluctant to dissociate and can break down calcium phosphate. This reaction is relevant not only to calcium phosphate in soil but also to calcium phosphate in teeth enamel. By means of orthosilicic acid, enamel is subject to slow and progressive degradation. “
This is a new one on me – and I spent many years researching the dissolution of apatite (the natural calcium phosphate) in soil and the factors influencing that. This arose because unacidulated phosphate rocks were being used in New Zealand agriculture. These material contain insoluble phosphate – in contrast to superphosphate which contain soluble phosphate.
The phosphate rocks used are mainly apatites and are complex (because of isomorphous substitution). A specific chemical equation for their dissolution depends on composition and environmental pH. But, in general, acid (H+) reacts with the apatite to produce Ca2++ H2PO4–, H2O, F–, Cl–, CO2, etc.
H+ + Ca10(PO4)6(OH,F,Cl)2 → Ca2+ + H2PO4– + H2O + Cl– + F– + CO2
Dissolution is promoted by the presence of acid (H+) and removal of dissolution products (particularly Ca2+). The calcium in soil solution can inhibit apatite dissolution – it drives the equation above to the left. The later is important because New Zealand agricultural soils have relatively high levels of calcium. On the other hand, our research showed that when soils are leached to remove calcium this can promote dissolution of the natural fluorapatite in the soil. (Perrott and Kear 2004). Removal of calcium from solution drives the above equation to the right.
Apatite particle size, fluoride content and substitution of other species in the apatite structure can also influence the dissolution rate of these materials in soil. But silica, or silica in soil solution – that is a new one on me!
Pity Sauerheber didn’t give a citation to support his claim that silica “has been long used in agriculture to break down solid calcium phosphate Ca3(PO4)2, thereby releasing soluble phosphate ion in soils even at neutral pH, for uptake by plant life.” I would be very interested to see the evidence – but I cannot find anything in the scientific literature to support Sauerheber’s statement. It appears to be a figment of his imagination and anti-fluoride bias.
In the same unsupported manner, Sauerheber is suggesting silica (“orthosilicic acid”) may be dissolving our teeth. He even provides a chemical equation for it:
2H4SiO4 + Ca3(PO4)2 → 2HPO4-2 + 2H3SiO4– + 3Ca2+
First, the primary mineral in teeth is a bioapatite (Ca10(PO4)6(OH,F,Cl)2) not Ca3(PO4)2. And H3SiO4– is not stable at the pH of drinking water or saliva so his idea is destroyed by the immediate reaction:
H+ + H3SiO4–→ H4SiO4
In other words, Sauerheber’s equation above is driven to the left at the neutral and acid pH values of saliva and drinking water.
Incidentally, it is the presence of Ca2+, H2PO4– and F– in our saliva (derived from food and drink) that drives the dissolution equation for apatite to the left. It prevents dissolution (acid attack or demineralisation) and promotes remineralisation. This the surface or “topical” mechanism that reduces decay in existing teeth when fluoridated water is used.
Sauerheber’s confusion
Sauerheber’s arguments are chemically confused – probably because he is driven by a wish to find anything connected with fluoride to be bad. He is confused by terminology because the silica in solution is often called orthosilicic acid, or silicic acid. But the point is that this species (whatever it is – the chemistry of silica in water is very complex) is not dissociated at neutral pH values near 7. (more correctly only 0.18% of it is – Belton et al., 2012). It is a very weak acid -significant dissociation to form the anion only occurs at higher pH values according to the equation:
OH– + H4SiO4 → H3SiO4– + H2O
Enamel attack is caused by acid (H+) not an unionized silica species or silicate anion. At these high pH values, dissociation of silicic acid at high pH does not produce H+. It actually removes OH–.
The same confusion is behind Sauerheber’s assertion that leaching of lead from pipes and plumbing is caused by “orthosilicic acid.” He says:
“it is the intact orthosilicic acid, the predominant form present over the pH range 7-10 (sic) that is leaching lead or lead salts from pipes and plumbing fixtures.”
In fact, acid in drinking water is one of the causes of lead leaching. The chemical species responsible is H+ and that is why treatment plants adjust pH levels to reduce acidity. Silica in solution does not make a contribution to the (H+) concentration.
But if it did then we should be concerned about all water as fluoridating chemicals make only a minuscule contribution to silica in water.









When you start running out of original arguments, Any port in a storm
LikeLike
It’s not so much runnung out of arguments as much as it is: ‘any argument will do.’
It’s a telling characteristic of any mindset that starts with a conclusion and then searches for justification. And yes, as one argument is trashed they’ll just grasp another, it never ends.
Pathetic really.
LikeLike
The laughable thing about this is that Silica is one of the major components of the water fluoridation additive. If there was any merit to any of this, the potential dangers of silica would have been one of the first, primary anti-fluoride arguments. . . And we are just hearing about this now?
It looks like Richard Christie is correct. Any argument will do.
Regarding Sauerheber, he is a con man and not to be taken seriously. This is from a correspondence that I had with him regarding one particular claim that he had made:
“Dr. Sauerheber,
I would like to ask you about this quote from Item 2, (early on in) this text: http://www.fluoride-class-action.com/wp-content/uploads/Sauerheber-95-letters-to-FDA-11-10-15.pdf
“It has come to my attention, from an attorney who is expert on Federal drug law, that the FDA-EPA MOU Memorandum of Understanding of 1979 (described in your response letter 2010) was officially revoked by the EPA in 1988. This was also confirmed by several other attorneys who recently published a summary of litigation filed in Los Angeles . .”
Could you please provide specific and accurate documentation proving that the 1979 MOU no longer exists?”
In response, he sent me the full text of his letter to the FDA (from where the quote came). So I asked him again:
“Thank you again for your replies.
Regarding this: ” in 1988 EPA published in the Federal Register that it terminated the agreement it made in 1979 (1979 MOU) with FDA to regulate water additives. This was effective in terminating the 1979 MOU (53 FR 25586-89 to be forwarded later).”
Can you please provide the text of what the EPA published in the Federal Register in 1988 regarding this issue?”
And in the next email: “There are many written materials out there claiming that the FDA has no authority over public drinking water and that the EPA does, but these claims have no meaning. Substances used for medical treatments are out of the jurisdiction of the EPA. The Office of Water wrote this several times to me and the MOU termination in the Federal Register are very clear. ”
Ok, so whatever is in the Federal Register is “very clear,” so eventually, with no straight answer from him, I looked up 53 FR 25586-89 that he had referenced earlier. So I asked him again:
“As of yet I have seen absolutely nothing in support of your claim that the 1979 MOU was terminated in 1988.
You did give me this: ” in 1988 EPA published in the Federal Registerthat it terminated the agreement it made in 1979 (1979 MOU) with FDA to regulate water additives. This was effective in terminating the1979 MOU (53 FR 25586-89 to be forwarded later).” Here, you are referring to this document, correct? http://www.fluoride-class-action.com/wp-content/uploads/53-FR-25586.pdf Have you actually looked at this document? Please point out for me the exact place where the dissolution of the MOU is addressed. Yes, the MOU is mentioned, as is the FDA, but after reading every word, I can safely say that this document has absolutely nothing to do with an alleged termination of the MOU in 1988. It is instead about the EPA outsourcing some of its responsibilities to NSF and the private sector, which was formalized in 1988. . . ”
After a lengthy diatribe into other issues, and without addressing this specific question, he ultimately ended his correspondence to me with: “Since you can’t even see this, there is little hope of this discussion having any meaning to you whatsoever (and vice versa).”
This is Richard Sauerheber.
Regarding the Open Parachute article, while I don’t think it’s a bad idea to debunk every moronic claim that surfaces, you are giving Sauerheber undue credit by taking him seriously enough to devote that much time to him.
LikeLike
The issue is far more complex than stated in this WordPress piece. The original notion that industrial silicic acid from silicofluoridation may be an ingredient that contributes to lead leaching from plumbing fixtures known to occur NY direct experiment is based on the fact that upside ion does not leach lead and theoretic studies by Urbansky and shock that silicouoridatex complexes which complex lead were thought to be neivuvke. Icicles acid is the only ingredient left in substantial quantities to be involved aso g as the pH is kept alkaline. Natural silicate polymers in water are u reactive. The silicic acid that is formed when industrial hazardous waste unsolicited acid is diluted into tap water which is alkainized with caustic sods is not natural silicate. The fact that silicic acid is used to ionizes calcium phosphate was published in an agriculture textbook. I never published the monograph that this WordPress piece attacks, because I have no direct experimental data on it. This is an editorial that attempts to explain the possible mechanisms by which silicofluoudation leaches lead from Pb rich umbing. I know have learned that Urbansky has published that it is S indeed possible that someiw level silicouoridatex could remain in treated waters under certain conditions the best operating theory us that these complexes plus industrial net formed silicic acid that is not polymeric plus any significant pHowering that occurs when the waste us added without sufficient caustic soda to neutrino it may all be contributing to the leaching er know occur. When lead is available. The leaching from uorixation is small compared to that seen in Flint due to over chlorination, but nevertheless is an issue since yoridationisys expect everyone to drink this material daily for life.
In Carlsbad sikucofluirudatiob caused school drinking king fountains to leach Pb to 50 ppb, even here in low lead CA. Many people font fate about 50 when typical levels in Flint are 1000, but 50 exceeds the MCL and we will never get to the official MCLG of zero by silicofluoridating water. Fluoridation is illegal, harmful, and useless.
F does not remineralize enamel. Normal enamel, like normal uncontaminated fresh pristine drinking water, contains no fluoride. F is not a mineral nutrient (neither us silica, natural or indusrrial). F does not incorporate into the enamel matrix but certainly incorporates into bone hydroxyapatite where it is a contaminant that stimulates formation of none of poor quality
I do not wish to conduct experiments to delineate he precise mechanisms by which silicic cofkuoridating leaches lead., because fluoridation of people is harmful, illegal, and useless, whether people have zero lead pipes or not. We can speculate for years to come the precise Pb leaching mechanism. But who would care? Understand?
LikeLike
Thanks for your comment Richard S.
Could you answer the following questions?
1: You claim the silica formed on hydrolysis of fluorosilicates is “not natural silicate.” Please explain the difference. I have shown that amounts of reactive silica in water are much higher than the amounts resulting from fluoridation – for both fluoridated and unfluoridated water. Please explain why you think there is a difference.
2: You say “The fact that silicic acid is used to ionizes calcium phosphate was published in an agriculture textbook.” Please cite the specific text book. As I said, as a researcher in this specific area of dissolution of apatites in soil I have never come across this claim and cannot find it in the scientific literature. So please cite the text your refer to so I can check it out.
3: You claim “Normal enamel, like normal uncontaminated fresh pristine drinking water, contains no fluoride. . . F does not incorporate into the enamel matrix.” Can you please provide citations for these claims as all the literature I have read says exactly the opposite?
LikeLike
David, I agree one should not give Sauerheber credit where it is not due. But really this article is not about him.
FAN and the anti-fluoridationists in general, are currently promoting the idea that fluoridation causes leaching of Pb from pipes, etc. They do not have a sound justification fo this but are giving it priority – together with their IQ claims. Unfortunately this is the “big lie” approach and does get traction. How often do we have to confront the knee-jerk claim that fluoride is a “neurotoxin?”
So I am doing a few articles on this Pb question to show their claims are unfounded. Sauerheber only comes into it because FAN etc., are promoting him as the expert on these issues. He is being quoted by activists and even this silly idea about silica has been promoted locally.
I asked Richard S. to respond and welcome his contribution purely because I believe that openness on this question is the best disinfectant. If he can substantiate his claims I will be happy to concede. If he can’t then hopefully a few activists will realise that using his claims will just make them look silly.
LikeLike
David – you say:
The really laughable thing is that silica (reactive silica, silicic acid, etc.) is a normal and natural component of drinking water – especially bore and surface waters. It is present in far higher concentrations that can be contributed by water treatment chemicals.
So if there really was a problem with silica – it has nothing to do with fluoridation and we should surely have known about it ages ago. This really is a figment of Sauerheber’s imagination and I think he may have conceded that in his comment.
LikeLike
Ken, Richard Sauerheber wrote
I do not wish to conduct experiments to delineate he precise mechanisms by which silicic cofkuoridating leaches lead., because fluoridation of people is harmful, illegal, and useless, whether people have zero lead pipes or not
I think the chances of Sauerhebe arswering questions about differences between natural and non natural silicates and mechanisms of dissociation/leaching, etc, are about zero.
He doesn’t care to understand, he just, …. you know, …he just knows.
The methodology I described in my first comment above is gloriously confirmed in this quote.
LikeLike
I’d also appreciate it if somebody could identify the language RS is using in the bulk of his comment above.
Bearing in mind that Google translate doesn’t translate gobbledegook.
LikeLike
Richard Sauerheber has been peppering me with emails. I have told him to send his comments here so there can be a public discussion (there is no point in me duplicating arguments in a private email discussion) but he doesn’t seem willing to do so. I have responded by telling him I will post his relevant comments here and provide responses here.
Here is one of his comments:
I am happy to remove his photo – I cannot see what the problem is (I obtained his photo from Mom’s against fluoride) or understand his sensitivity. But no problem – easily removed.
No one pretends blog articles are peer-reviewed. But so what? neither was Sauerheber’s article peer-reviewed. I cannot understand why he sees participation in a discussion about chemistry as suicidal. Nor why he is afraid to participate in a discussion with “fluoridationists.” I willingly participate in discussions with “anti-fluoridationists” and would do it more often but they usually ban scientists from participation in their forums.
Later on, I will devote a comment to the acid/base reactions in water and include some more of Richard S.’s email comments about that.
LikeLike
The “article” being discussed is not a published article, it is an editorial of opinion and deductions that attempts to explain how it is possible that fluosilicic acid treated water leaches lead when lead is available on plumbing. Discussions among water treatment professionals indicate that typical water containing natural silica have most silicon on the firm of polymeric silicates that is unreactive. There can typically be reactive silicic acid at levels less than 1 ppm in water with silicates. So industrial silicic acid formed in situ is a strong candidate to be able to leach lead ion particularly from lead carbonate or phosphate. The article appears to state this strongly and should be amended to state this as a possible contributing mechanism. To me it is this, plus siciofloride complexes, plus lowered pH in general that may all contribute to the lead leaching caused by fluoridation.
LikeLike
Thanks Richard S. You have confirmed that the ideas in your article are your own speculations and do not have evidential support. No objection about that – but an honest proposer of a speculative idea should welcome discussion. Unfortunately, FAN and anti-fluoride campaigners are using articles like this as evidence – and you promote that. So a responsible scientist has an obligation to challenge your speculations and expect you to participate in an honest debate of the.
Yes, the nature of silica in water is complex. While it is useful to talk about “orthosilicic acid” in fact olation and oxalation reactions proceed and various degrees of polymerisation are expected. Eventually, this polymerisation process leads to precipitation of a silica solid or gel form of SiO2. This is true whatever the source of silica – “natural” or hydrolysed fluorosilicates.
For practical purposes silica in solution is analysed by reaction with molybdate and is defined as “reactive silica” This gets around the problem of vagueness about the actual chemical species present. Abd, after all, it is the reactive form we are interested in – even if this includes some dimeric or oxolated forms.
The data I present in this article are for “reactive silica.”
It is the normal species measured by water chemists. I suggest you have a look at the chemical analysis reports for the water provided by your local utility – I am sure you will find that this is how it is described. And I bet the concentrations are much greater than the 1 ppm yiou claim.
While we are at it – can you provide a source or citation for your claim that reactive silica naturally in water is always less than 1 ppm?
OK, I think you have acknowledged that the ideas in your article are only speculative -even though you present them as fact. Hopefully, you will amend your article to make this clear.
But, please, if you must speculate then you should be prepared for a critical discussion. That is the normal scientific process.
I am never opposed to speculation. But I am opposed to people claiming their speculations are factual. And I reject the hubris of people who insist on presenting their speculation in this manner while refusing to allow discussion or criticising those who do discuss the ideas.
LikeLike
Richard Sauerheber has made the following comments about silica in drinking water to me by email:
Ok, he sees a difference between clean fresh water and pristine water. Pristine means something along the lines of “having its original purity; uncorrupted or unsullied.” So clean fresh water from the treatment plant is not “pristine.
“
I get that – but it makes no difference. He should look up the chemical analyses of samples form “pristine” sources – like his Carlsbad well.
I cannot find a report for his Carlsbad well but online it describes it this way:
LikeLike
Pingback: Prime-time Dishonesty from Anti-Fluoride Group | Zeitung für Katzen
While I may one of those opposed to fluoridation, primarily on the grounds that it violates the Nurenburg Codes of 1947 with regard to informed consent in any medical procedure or experimentt, and fluoridation of public water supplies is a medical experiment on an uninformed public. However, the argument that silicic acid is a danger is simply incorrect. Biologically available silica is an essential nutrient in small amounts. It plays roles in collagen and bone formation and maintenance and detoxification of aluminum which is a true neurotoxin. Dr. Christopher Exley of Keele University has demontrated that consumption of silicic acid rich drinking water increases excretion of aluminium as hydroxyaluminosilicates. It appears that soluble silica consumed in water can enter cells, even cross the blood-brain barrier and react with any aluminum present, forming the hydroxyaluminosilicates that the cell then excretes as a waste product into the blood stream to be removed by the kidneys. The significance of this is that Dr. Exley treated 8 patients with Alzheimer’s with natural spring water with high contents of silicic acid (approx 30 ppm), and it reversed the Alzheimer’s in 3 patients. Dr. Dale E. Bredesen, an Alzheimer’s researcher at the Buck Institute for Research on Aging in Novato, CA, USA has found a very similar result in his 37 step protocol for reversing cognitive decline related to early to middle stage Alzheimer’s. The protocol was successful i reversing cognitive decline in all but one of the initial 10 patients. The patient who did not recover had more advanced Alzheimers with significant structural brain damage. .In his initial tests of the protocol 3 of 10 patients who tested positive for high levels of toxic metals (Al, Hg, Pb, Cd) reversed their cognitive decline by undergoing a metal detox protocol. His work is published in the journal ‘Aging’ (2014 and 2016 papers) and in the book, ‘The End of Alzheimer’s” (August, 2017). Fluoride in the public water supply is relavent to this issue because fluoride increases the solubility of aluminum and alumium salts are often used as flocculants in treating public water supplies. Aluminum’s neurotoxicity is well established (at least two books were published on the subject in the 1950’s) and the EPA has published exposure limit of 5 mcg/kg body weight for aluminum administered intraperitoneally, but appears to have no standard for aluminum in drinking water. The issue of aluminum exposure and its relavence is contentious because most vaccines contain aluminum salts as an adjuvant and attempts for find equally effective alternative adjuvants have been relatively unsuccessful. Consequently, Dr. Exley’s work is villified by vaccine advocates who claim Dr. Exley is attempting to villify aluminum and therefore vaccines (see: https://respectfulinsolence.com/2017/11/29/christopher-exley-using-bad-science-to-demonize-aluminum-adjuvants-in-vaccines/). However, Dr. Exley’s work (see: https://www.sciencedirect.com/science/article/pii/S0946672X17308763), questioning the role of aluminium adjuvants in neurological and autoimmune disorders.
LikeLike
Kenneth, it is interesting that complexing aluminum with silica is being suggested as a mechanism for ridding the body of Al. Fluoride also complexes with Al, so perhaps it also reduces Alzheimers?
Al salts are used to flocculate organic matter etc., in water treatment – in the processes Al is removed from solution. Have a look at the chemical analysis data for tap water and you will find Al levels are usually below the detectable levels. Of course, fluoride in the source water will be reduced by the Al treatment (removed in the flocs) and fluoridating chemicals are only added at the end of the treatment process when Al is no longer present.
LikeLike