It’s easy to find studies that confirm ones own bias on health issues. But cherry-picking such studies is unscientific because one could equally cite studies showing the exact opposite. The problem is these studies often rely on poor data, do not sufficiently account for confounding factors and often use poor statistical analysis so contradictory results are inevitable. A more objective requires a meta-analysis of all the available studies.
Anti-fluoride activists do this when they cherry-pick studies to argue that science has “proved” fluoride intake by pregnant women lowers the IQ of their children. These activists rely on just a few studies from two related groups – they cite Bashash et al (2107), Green et al (2019) and Till et al (2020). But what about studies that show the exact opposite – an increase in child IQ and the low fluoride intake relevant to community water fluoridation. Studies they ignore like those of Santa-Marina et al (2019) and Ibarluzea et al (2020).
The publication this month of new extensive data from an important study will be harder to ignore. This establishes a relationship of prenatal maternal intake of fluoride with the cognitive results for children in areas of Spain where community water fluoridation is used. In contrast to the negative results found in the studies anti-fluoride activists rely on, this new study reported: “Maternal fluoride levels were associated with better cognitive scores in childhood.”
Here is the citation for the new study:
Ibarluzea, J., Gallastegi, M., Santa-Marina, L., Jiménez Zabala, A., Arranz, E., Molinuevo, A., Lopez-Espinosa, M.-J., Ballester, F., Villanueva, C. M., Riano, I., Sunyer, J., Tardon, A., & Lertxundi, A. (2021). Prenatal exposure to fluoride and neuropsychological development in early childhood: 1-to 4 years old children. Environmental Research, 112181.
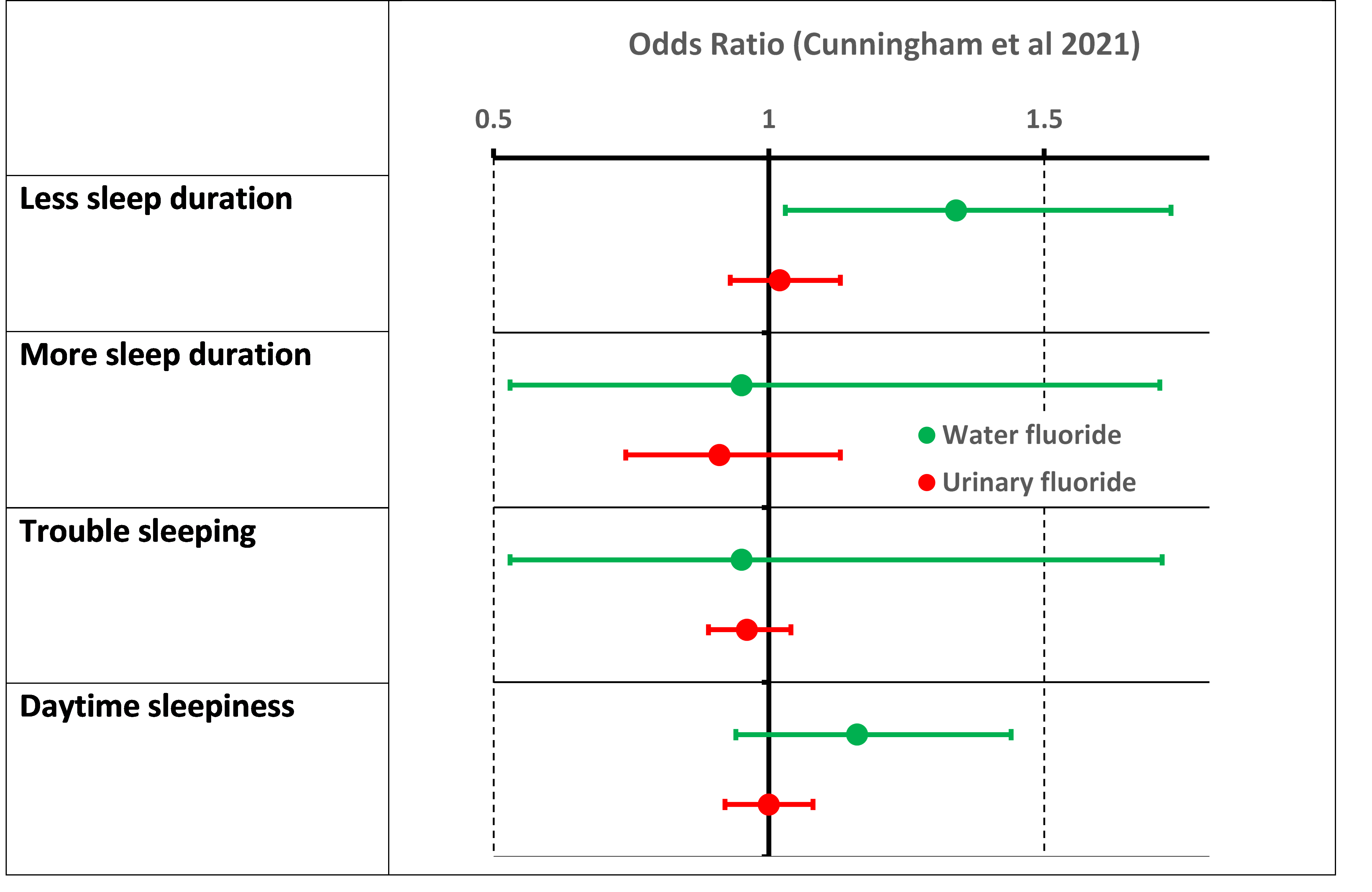
The figure below summarises the results for relationships of child cognitive scores with maternal prenatal urine – a measure of fluoride intake. cognitive scores are from McCarthy Scales tests appropriate for age 4. GCI is a General Cognitive Index.
The data points are for ß coefficients of the linear regressions of cognitive score against material urinary fluoride – the slope in units of amount of cognitive change per unit of urinary F (mg/L or equivalent). The bars are the 95% confidence intervals – if they include zero in the range this indicates the score is not beta value for the score is not statistically significant.

As we can see all the cognitive scores a positive and statistically significant for boys – hence the conclusion that “Maternal fluoride levels were associated with better cognitive scores in childhood.” However, none of the changes in scores for girls was statically different from zero
Don’t get me wrong. I am certainly not arguing that maternal intake of fluid will increase the cognitive levels of offspring male children. I am not attempting to confirm a pro-fluoride bias here. Remember, I said that objective assessment requires a meta-analysis of all the available studies.
The figure below shows the results of a meta-analysis of all the available studies which considered the relationship of the cognitive score with prenatal maternal urinary F.
As we can see, some of the beta values are positive and some are negative. Many are not significantly different from zero – any effect is not statistically signficant. Over all the studies, on average the score is positive (ß = 2.1 for 1 mg/L increase in urinary fluoride, or the equivalent measure) but not statistically significant from zero (95% confidence level is -1.9 to 6.1).
So the best objective conclusion to date is that child cognitive scores are not influenced by prenatal maternal fluoride intake at the levels expected in these studies which are relevant to community water fluoridation.
Conclusion
These studies illustrate the danger of cherry-picking studies to draw conclusions. or even simply taking the data from only a few studies that have so far been published. But that is what Grandjean et al (2021) have done in the benchmark analysis of cherry-picked studies. They concluded that a safe level for maternal urinary fluoride is as low as about 0.2 mg/l.

Image credit: Cherry-Picking
At best they counted their chickens before they had hatched, given that some important studies had not been published in scientific journals. At worst the cherry-picked to confirm a bias.
I suspect the latter because the important Spanish data had been published as conference papers so they should have been aware of them. The authors of the benchmark paper are all associated with the limited number of studies they considered (particularly those of Bashash et al (2107), Green et al (2019) and Till et al (2020)) and were members of just two groups obviously coordinating their work. Authors from this group have been very active in promoting their own findings, studiously ignoring the findings of voters and also using their work to promote the arguments of anti-fluoride activists against community water fluoridation.
Just imagine if some pro-fluoride activists chose to claim that fluoridation will actually increase child IQ and cherry-picked the Spanish studies to support their claim. I can’t actually see that happening, but if it did it would be as bad as the approach used by the authors of Grandjean et al (2021).
This tactic of cherry-picking studies may be used to provide a scientific basis for activist arguments – but that is not true science.











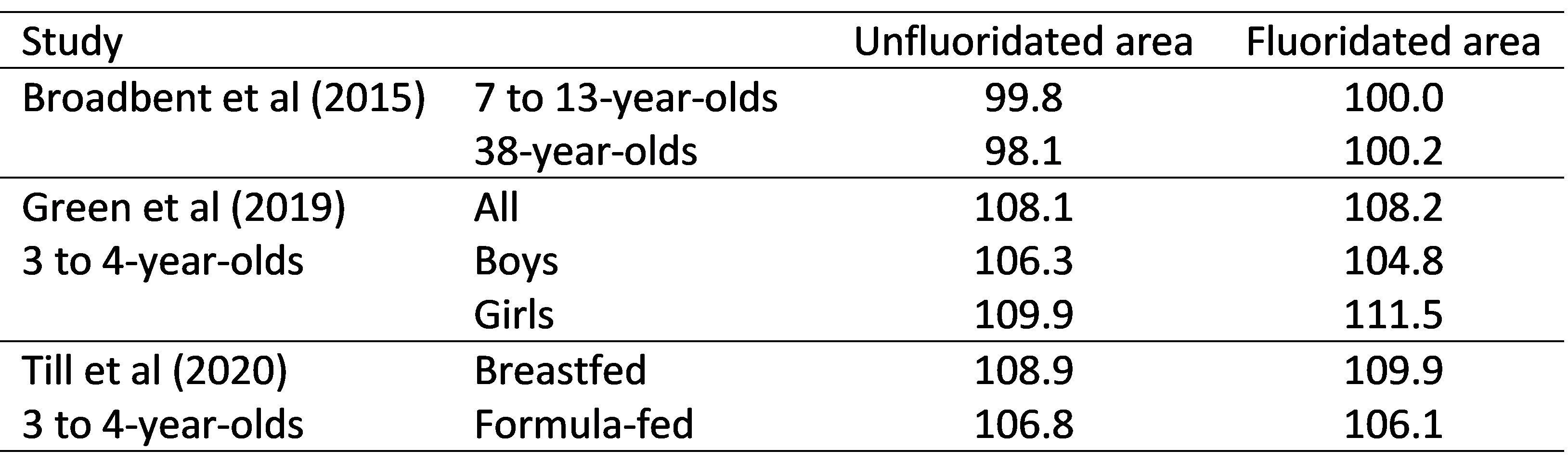 I think this comparison should have been made clear in the review because it is important but is most often overlooked because opponents of fluoridation, and the study’s authors, never consider it. They remain silent about the facts in this table. This is hypocritical considering the attempts anti-fluoridation critics made to discredit the same finding reported by Broadbent et al (2015) when their paper was published.
I think this comparison should have been made clear in the review because it is important but is most often overlooked because opponents of fluoridation, and the study’s authors, never consider it. They remain silent about the facts in this table. This is hypocritical considering the attempts anti-fluoridation critics made to discredit the same finding reported by Broadbent et al (2015) when their paper was published.